Match to Autism Clinical Trials
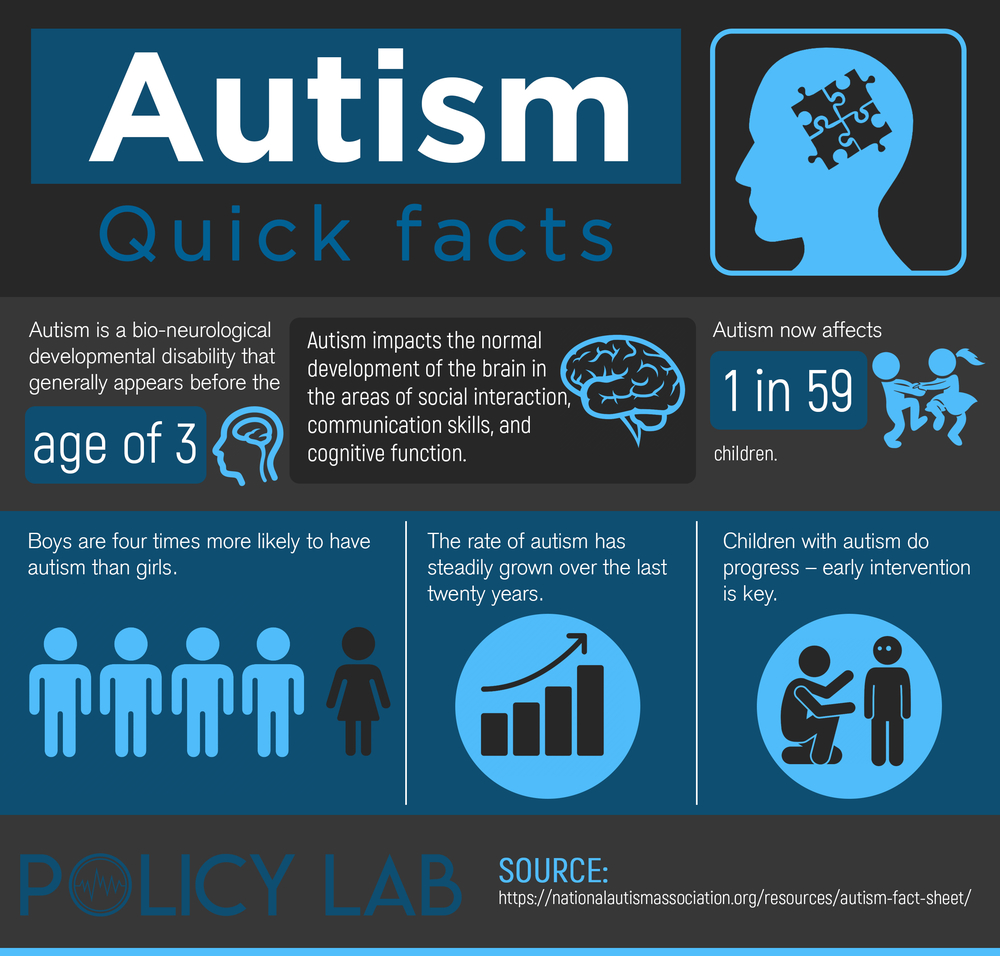
Autism spectrum disorder is an umbrella term for a wide range of conditions primarily characterized by difficulties with communication, and marked behaviors such as repetition (of words or behaviors); social withdrawal lack of interest in relationships; and in some cases speech impairments.
The Centers for Disease Control and Prevention estimate that 1 in 59 children fit in the criteria for autism spectrum disorder, and it is four times more common in boys than in girls. There are no known causes for autism, however research has suggested that genetic factors seem to play a role in the development of the condition.
What is a Clinical Trial?
Clinical trials are designed to test interventions such as new medical devices, lifestyle changes, or medications. After many years of empirical and laboratory research, scientists and doctors design a clinical trial in order to determine if their intervention is safe and effective for their target population.
Clinical trials are separated into the following phases:
Phase 0
At this point, scientists are interested in learning how the medication works, and to do so they recruit a very small number of volunteers. Usually, a group between 10 and 15 individuals will test the medication to determine if the drug is safe in humans.
Phase I
The aim of phase I is to determine how the drug works in the body for an extended period of time. Phase I is usually conducted in healthy individuals and the group tends to be larger than in phase 0.
Phase Ii
After the medication was deemed safe in phase I, it is ready to be tested in the target population. This means that if the new intervention is aimed towards individuals with autism, in phase II researchers will look for individuals with autism.
Phase Iii
During this phase researchers need to recruit a large number of individuals (3,000 or more) and will compare the intervention tp already-existing standards that are used for treating the target condition. For example, if researchers are developing a new headache medication they will need to compare it to what has already been demonstrated to work for headaches (i.e. ibuprofen) to determine if the new treatment at least as effective, safe, and accessible as the current standard.
Phase Iv
This phase is usually ongoing and occurs after the drug has been approved and it is available to the general population. Many years after a treatment has been released, researchers have to continue gathering information about the safety and effectiveness of the intervention.
What Are My Rights/My Loved Ones Rights As a Clinical Trial Participant?
Clinical trials are completely voluntary, that means that even if your doctor offered you or advised you or your family member to enroll in a clinical trial the decision is always yours.
All study participants/legal guardians have the right to decide whether they want to participate or not in a clinical trial. Even if you agreed to participate at first you have the right to withdraw your consent at any point during the study.
Typically, the information that researchers gather from a clinical trial is completely confidential, and you have the right to keep it that way. Before you or your loved one enrolls in a clinical trial, the research staff will ask you to fill out an informed consent form that will explain how they plan to keep your information safe and confidential.
Why Is Autism Research Important?
Currently, there are no drugs or interventions available that effectively treat or cure autism spectrum disorder. However, researchers are working tirelessly to find an intervention that can help individuals with autism and their loved ones cope better.
Clinical trials are a critical part of that process. Without clinical trials researchers would not be able to test new interventions and determine what works what doesn’t on people with autism.
Many times the outcome of a clinical trial is that the intervention was not effective, however, this does not mean that your participation was in vain. Even if you didn’t receive a direct benefit, when you or your loved one decides to participate in a clinical trial, you are helping scientist develop better and more effective treatment and interventions that can significantly improve the quality of life of future generations.
Autism Clinical Trials Looking For Participants
Match to Autism Clinical Trials
Sources
- Autism Spectrum Disorder Data & Statistics. Retrieved from: https://www.cdc.gov/ncbddd/autism/data.html